One of the first real challenges you will have is to pass thermodynamics class. When you start taking classes such as Thermodynamics, you’re taking “gatekeeper” courses. The courses that gatekeeper you from graduating and becoming a mechanical engineer.
I remember spending long nights past 1 am working on thermodynamics homework. My professor told me he was impressed that I was up at 1 am doing homework. I remember this even 10 years from now. I sent countless emails to my professor to ask questions about homework, labs, exams, etc. Get in the habit of asking questions as soon as you have one, provided that you have exhausted all of your resources.
My goal with this guide is to make Thermodynamics simple for you, because thermodynamics is a very hard class. Engineering professors, although smart, are not the best ones to explain thermodynamics in simple terms. I won’t be able to teach you how to do problems entirely, but use the framework below to create an understanding.
You will need to refer to your examples and your homework problems over and over again until you understand. This will likely take hours of your time, just a warning.
Mechanical Engineering is really challenging – see 8 Tips On How To Pass Mechanical Engineering
February 29, 2024Fundamentals of Thermodynamics
First Law
Conservation of energy in a system means that energy cannot be created or destroyed, and that it is only converted.
Defined by the formula:

You need to know what kinetic and potential energy is from your Physics class.
Second Law

The Second Law of Thermodynamics states that entropy in a system must always increase. Entropy refers to disorganization in a system.
For example, let’s picture a bottle filled with water. We empty out the bottle, and then pour the water back in. If we look at the water at a microscopic level, we see that the particles containing the water would be arranged different. It would be impossible to pour the water back in and achieve the same state as we had initially.
Third Law

Entropy of a systems approaches an absolute value as its temperature approaches zero.
Power Cycles
Power cycles consist of thermodynamic processes that involve the transfer of heat and work both in and out of the system.
The input being the amount of heat put into the boiler and the output being the amount of work done out of the system through the blades of the turbine.
Power plants and engines utilize the theory of power cycles behind their application.
Carnot Cycle
The Carnot Cycle is the ideal thermodynamic cycle, assuming isothermal/isentropic expansion and compression.
Isothermal means that the temperature in the process stays constant. (Delta T = 0)
Isentropic meaning both adiabatic and reversible. When referring to a process that is reversible, the s values are the same. See the above figure for reference.
Adiabatic meaning a thermodynamic process without transferring heat. (Q = 0)
Reversible meaning that the process can be returned to its original state. Think back to the water bottle example.
Rankine Cycle
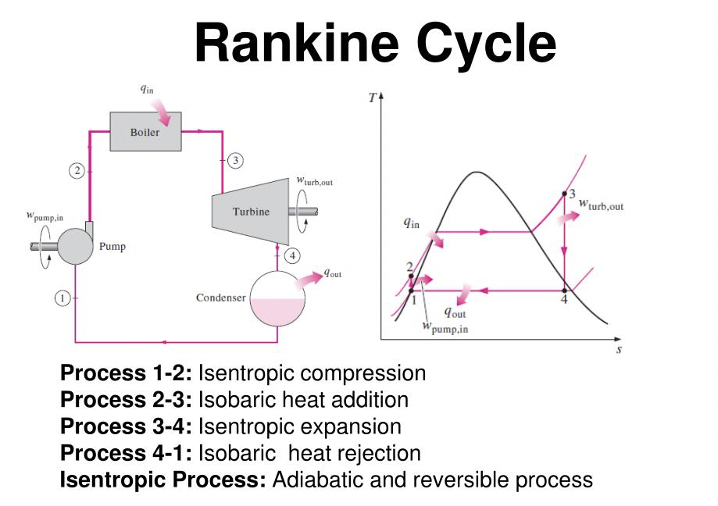
As covered in my interview guide. This is the most applicable cycle to the real world. You should be able to understand this, especially as an engineer. This isn’t even about book smarts.
Know how to answer questions about the Rankine Cycle in an interview. Interviewers will test real world understanding of thermodynamics.
Otto Cycle

The Otto Cycle is the ideal thermodynamic cycle describing the function of a spark ignited piston engine.
The Otto Cycle Processes Are:
0-1: Air is drawn into the piston at constant pressure, as you can see the P-value remains the same.
1-2: Adiabatic compression from bottom dead center (BDC) to top dead center (TDC).
2-3: Constant heat volume transfer, represents the air fuel mixture in an engine igniting.
3-4: Adiabatic expansion, relating to the power stoke.
4-1: Heat rejection from the air, while the piston is at bottom dead center.
1-0: Air is released to the atmosphere at constant pressure.
Power Cycle Problems
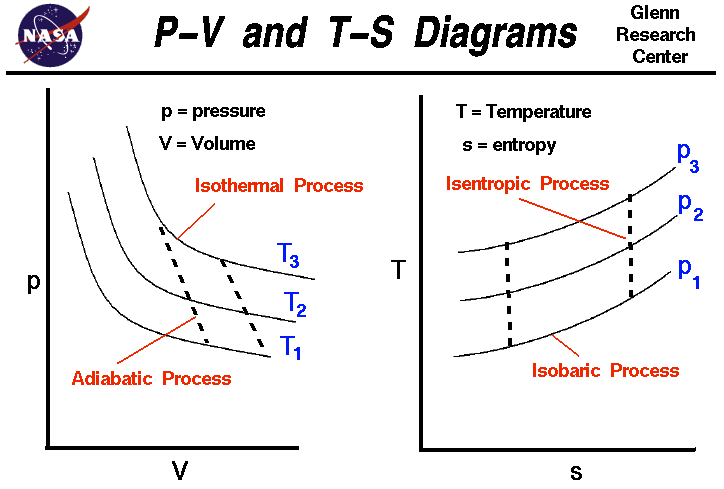
The problems regarding power cycles will refer to the T-S and P-V diagrams. Make sure you understand these diagrams, as you will be referring to them when you do your homework and exams. You also need to understand the various points on the T-S and P-V diagram.
You will also need to be able to look up thermodynamic values such as the enthalpy of refrigerant 134a and use those to solve for variables in homework and exam problems.
Make sure you can physically understand what saturation and superheated states of a liquid mean.
Studying to Pass Thermodynamics Exams
Review your textbook, practice example problems, homework problems, and review solutions over and over. This is all you need to do to be able to pass Thermodynamics class. Students make the class seem harder than it needs to be, but the class really isn’t, once you break things down and understand what is going on.
Thermodynamics Exam Questions
Thermodynamics test questions will likely be a spin off of your homework. Your professor will have a review and the problems in the review will be similar to what is on the exam. Make sure you understand each problem and think about variations in what the problems would ask. If you need to, go to office hours. The professor makes their time available for you to come in and ask questions, so take advantage of it.
Remember After You Pass Thermodynamics
It’s really easy to cram as much material as you can in your head for a class and then forget it shortly after, but this is a very important class.
Remember the laws of Thermodynamics and your power cycles. You will get asked about these in engineering interviews. These are also good concepts to understand when you socialize with engineers. Engineers are smart and know what goes on in various systems that we interact with everyday. You don’t want to come off as dumb or ignorant by not remembering thermodynamics concepts.
Applications of Thermodynamics
Common products that are applicable to thermodynamics include pumps, motors, fridges, ovens, boilers, turbines, and engines.
Closing
Thermodynamics is not easy, but it gets easier when you break things down into systems and understand the fundamentals.
Additionally, if you’re studying mechanical engineering and interested in thermodynamics, we offer career services such as
Hundreds of engineers have used our services to get 10X more interviews, $100,000+ salary jobs, and have been able to do so within a month.
About the author

Kazuyoshi Fujimoto, PE
Founder | Engineering Career Coach | Principal Mechanical Engineer
Kazu oversees all of ultmeche’s engineering services. He provides consulting such as resume reviews, rewrites, mock interviews, and all services career related. Additionally, Kazu performs consulting work regarding Oil & Gas, Automotive, and Aerospace & Defense. Kazu is licensed as a professional engineer in the state of California and has 9+ years of experience in Oil & Gas, Automotive, and Aerospace & Defense.

4 thoughts on “How To Pass Thermodynamics Class”