
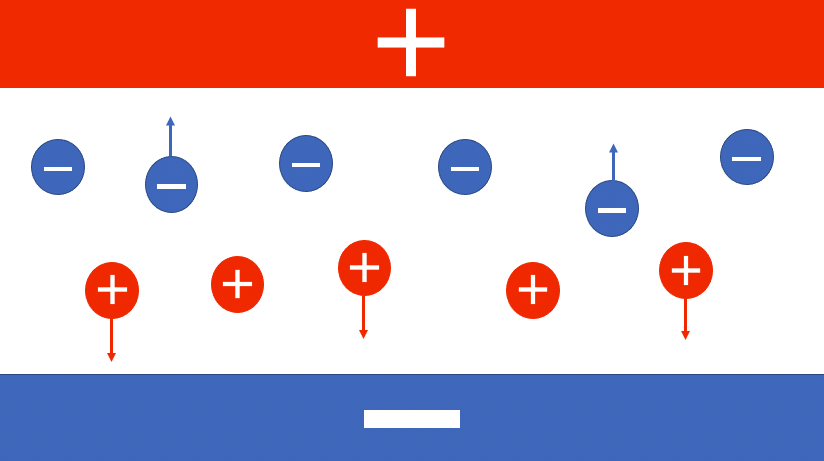
In my technical mock interviews, I always ask the question – What is galvanic corrosion? Simply put, galvanic corrosion is when two dissimilar materials are coupled in a corrosive environment. When we look at this in terms of metals being paired together, the less noble becomes the anode and the more noble acts as the cathode.
So, what does this mean? The materials you select are critical to your application and they won’t last forever. You’re going to responsible for material selection as an engineer so you’ll need to understand how materials react with certain environments.
Ultimately materials corrode after a long period of use. We want materials to have a long life, which is why we care so much. You do not want to be responsible for designing something, only for corrosion to occur shortly after. Save the company that you are working for some money by understanding proper selection.
February 29, 2024Galvanic Corrosion Chemistry Terms To Understand:
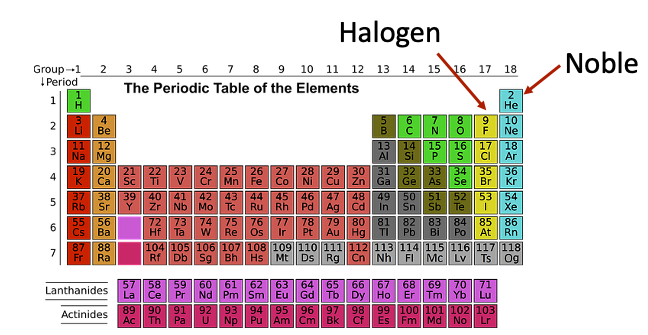
What does noble mean in chemistry?
Look back to your chemistry class and remember using the periodic table. Each column represents the amount of electrons each element contains. The noble gases consist of all of the elements of the very right of the periodic table.
The noble gases are Helium, Neon, Argon, Krypton, Xenon, and Radon. The column to the left of these elements are Halogens. These are very reactive because they need that one electron to become “noble” and fill their electron outer shell.
What is an anode?
An anode acts as an electrode in which the current enters the system.
What is a cathode?
A cathode as as an electrode where current leaves the system.
What are examples of corrosive environments?

Sea water and air are two examples. Structures in the ocean are a clear cut example of galvanic corrosion. When you design parts that go in the sea or air, you need to account for this. There is no avoiding corrosion. Think about leaving your metal bike outside for a few days. As a result of the metals reacting with the air, the bike starts to rust.
Why do we care about Galvanic Corrosion?
Galvanic Corrosion has a huge impact on our material selection and overall, the life of the part. If we don’t account for galvanic corrosion, we will see a large reduction in the life of the product you design as an engineer. As an engineer you need to be concerned with the cost and integrity of your part.
Why would Galvanic Corrosion be asked in interviews?
Engineering interviews expect you to have an understanding of physics and chemistry. This understanding does not have to be complex, but basic. You know you have an understanding of galvanic corrosion when you understand material selection and basic chemistry. This perspective is necessary when you start working as an engineer. Tunnel vision is a problem engineers in the industry face. Because of tunnel vision, engineers forget the outside perspective. As a result, mistakes are made.
FAQ
Do I need to know chemistry as an engineer?
Yes, at the very least you need to have a basic understanding of chemistry as an engineer. The systems you design are going to be subject to corrosive environments. This means you need to know what water or air will do to your parts.
Will Galvanic Corrosion be asked during interviews?
Yes, expect to be asked this question, especially if the position requires design engineering. There’s a good chance you won’t be asked about this, but its important to know in general.
What is a practical example of Galvanic Corrosion?
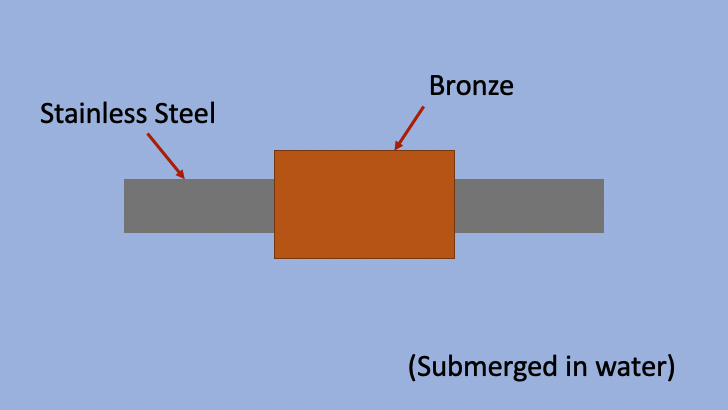
Let’s say you have a shaft paired with a coupling inside of a pump. Excuse the elementary picture, but you get the point. The material of the shaft is 416 Stainless Steel, and the coupling is ASTM B505 Bronze. (Hint, this is a horrible material selection combination, your shaft and coupling should be the same material) Because we are talking about pumps, this shaft and coupling is submerged in water. As we have stated above, water is a corrosive environment. The less noble of the shaft and coupling will give off electrons, and the more noble will take those electrons.
This will reduce the life of your pump, reduce performance, and cost more money. As a result, not accounting for corrosion will be expensive.
If you found the answers to questions like these useful, book a mock interview with me. During our mock interview, we will go over a series of general behavioral and technical questions that you should know. The technical questions aren’t good only in an interview setting, but are good to generally know as an engineer as well.
About the author

Kazuyoshi Fujimoto, PE
Founder | Engineering Career Coach | Principal Mechanical Engineer
Kazu oversees all of ultmeche’s engineering services. He provides consulting such as resume reviews, rewrites, mock interviews, and all services career related. Additionally, Kazu performs consulting work regarding Oil & Gas, Automotive, and Aerospace & Defense. Kazu is licensed as a professional engineer in the state of California and has 9+ years of experience in Oil & Gas, Automotive, and Aerospace & Defense.
